Subtitle Notification: The first and the second author have equally contributed to this project
Introduction: Transfusion-dependent β-thalassemia (TDT) confers substantial burden on both patients and the healthcare system, attributable to disease- and treatment-related complications due to the need for lifelong regular red blood cell (RBC) transfusions and iron chelation therapy (ICT). The present study primarily aims to determine the prevalence and severity of treatment- and disease-related complications in a representative sample of 200 TDT patients managed in routine care settings in Greece.
Methods: ULYSSES is an epidemiological, multicenter, retrospective cross-sectional study. Data were collected during a single study visit and through medical chart review. Adult patients with TDT [defined as having received ≥6 RBC units (excluding transfusions for elective surgery) and no transfusion-free period ≥35 days during the 24-week pre-enrollment period], diagnosed at least 12 months prior to enrollment, were consecutively enrolled. Exclusion criteria included diagnosis of hemoglobin S/β-thalassemia or α-thalassemia, pregnancy during the previous year and receipt of bone marrow transplant. Data from the pre-planned interim analysis on the first 100 eligible consented patients, enrolled from October 2019 to February 2020 by 10 major hematology departments, are presented.
Results: At enrollment the patients' median (interquartile range: IQR) age was 45.9 (39.4-49.0) years and 61% were females. Of the patients, 37% were employed, 34% had retired, 4% were unemployed, and 25% reported other employment status. Non-thalassemia-related medical/surgical history was reported in 59%. Regarding the patients' genotype, 48% were classified as β+β+, 37% as β+β0, 10% as β0β0, and 5% as β+/δβ-Sicilian or β+/HbLepore. The most common β-globin gene mutations were IVS1-nt110 G>A (71%), IVS1-nt6 T>C (24%) and Codon 39 C>T (21%).
All patients were receiving ICT whereas 54% had undergone splenectomy at a mean (SD) age of 23.1 (11.2) years; splenectomy was more common in older ages (30.8% vs 62.2% for patients aged 18-40 and >40 years, respectively).
The median (IQR) age at β-thalassemia diagnosis, at initiation of RBC transfusions and of ICT was 0.8 (0.4-3.0), 1.5 (1.0-5.0) and 5.6 (2.8-10.3) years, respectively. During the 48 weeks before enrollment, the median (IQR) average pre-transfusion hemoglobin levels were 9.9 (9.5-10.4) g/dL, the median (IQR) average serum ferritin levels were 464 (263-969) μg/L, and the mean (SD) total RBC units transfused per patient was 34.9 (10.2).
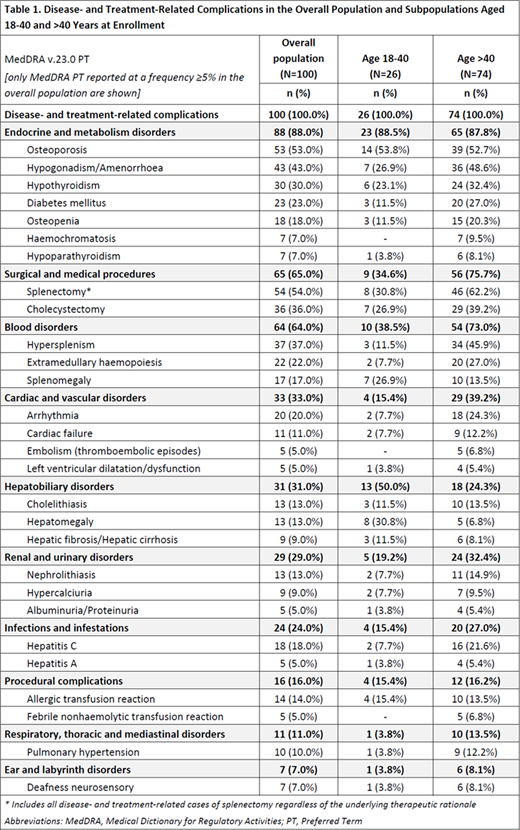
During the period between diagnosis and enrollment [mean (SD): 42.5 (7.1) years], patients had cumulatively developed 634 (median: 6 per patient; range 1-19) disease- and treatment-related complications (24.7% of Grade ≥3), of which 262 were ongoing at enrollment in 85% of the patients. Complications with a frequency of at least 20% included splenectomy, osteoporosis, hypogonadism/amenorrhea, hypothyroidism, diabetes mellitus, hypersplenism, extramedullary haemopoiesis, cholecystectomy, and arrhythmias (Table 1). All patients had experienced disease-related complications (523 in total; 25% of Grade ≥3); 43% had experienced 111 treatment-related complications (23.4% of Grade ≥3) with 73 being transfusion-related and 35 ICT-related. In the age group of 18-40 years, 26.9%, 19.2%, and 11.5% had experienced at least one treatment-, transfusion- and ICT-related complication, respectively. The respective rates in patients >40 years of age, were 48.6%, 37.8%, and 28.4%.
Conclusion: In this study, over an average 42-year period since β-thalassemia diagnosis, a median of 6 complications per patient were reported. All patients had developed disease-related complications and 4 out of 10 had developed treatment-related complications. About 1 in 4 complications were of Grade ≥3. The most frequent complications included endocrine/metabolism disorders, surgical/medical procedures, and hematological and cardiovascular disorders. The treatment-related complication rate was about double in patients aged >40 than in those 18-40 years at enrollment. These preliminary results underscore a considerable complication burden of TDT.
Acknowledgements
The authors acknowledge partial financial support from Genesis Pharma and Celgene Corporation and editorial support from Qualitis Ltd, sponsored by Genesis Pharma.
Kattamis:Genesis Pharma SA: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Vifor: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Agios: Consultancy; Apopharma/Chiesi: Honoraria, Speakers Bureau; Vertex: Membership on an entity's Board of Directors or advisory committees; Ionis: Membership on an entity's Board of Directors or advisory committees. Voskaridou:GENESIS Company: Consultancy, Research Funding; ADDMEDICA Company: Consultancy, Research Funding; NOVARTIS Company: Research Funding; PROTAGONIST Company: Research Funding; ACCELERON Company: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Delicou:Genesis Pharma SA: Research Funding; Novartis: Honoraria, Other: Investigator fees; Ionis: Other: Investigator fees. Klironomos:Genesis Pharma SA: Research Funding. Lafiatis:Genesis Pharma SA: Research Funding. Petropoulou:Genesis Pharma SA: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Investigator fees. Diamantidis:BMS: Membership on an entity's Board of Directors or advisory committees; Genesis Pharma SA: Honoraria, Research Funding. Lafioniatis:Genesis Pharma SA: Research Funding. Evliati:Genesis Pharma SA: Research Funding. Kapsali:Acerta Pharma: Research Funding; Amgen: Research Funding; BMS: Research Funding; Celgene: Research Funding; Novartis: Research Funding; Roche: Research Funding; Takeda: Research Funding; Teva: Research Funding. Karvounis-Marolachakis:Genesis pharma SA: Current Employment. Timotheatou:Genesis Pharma SA: Current Employment. Kourakli:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Vifor: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Protagonist: Research Funding; Celgene / BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Genesis Pharma SA: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; IMARA: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ionis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal